Trusted by the world's top research organizations









































































Scientific literature is growing exponentially.
Our AI helps you keep pace.
Turn hours of literature review into minutes while maximizing your content budget.
-
Ask AI
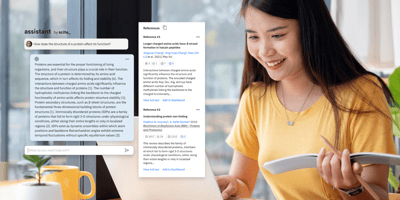
AI built for research
Instantly access research insights with our AI-powered Assistant. Leveraging over 1.2 billion Smart Citations, it provides evidence-based answers, streamlining literature reviews and helping you explore new topics efficiently.Learn More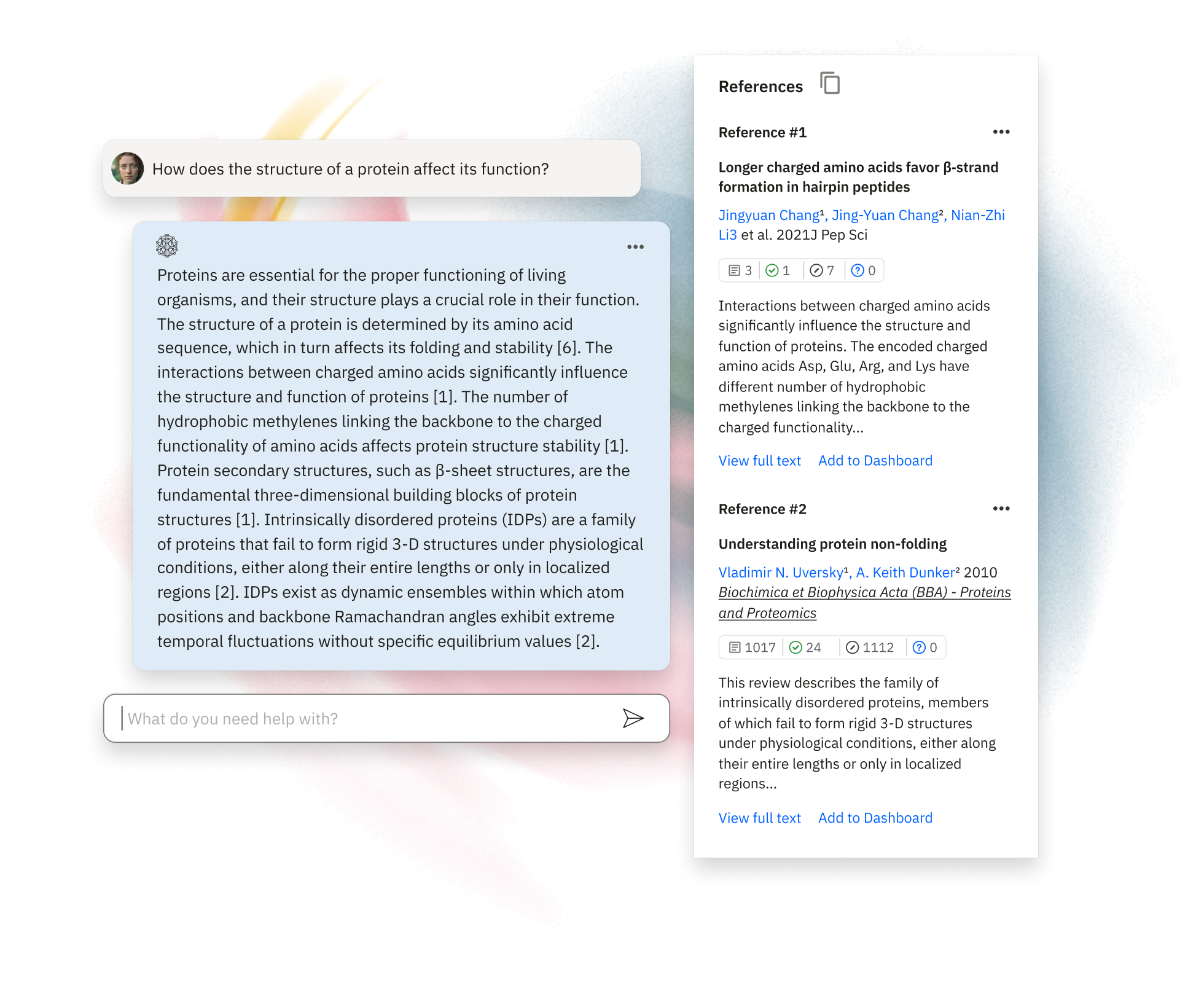
-
Full-Text Search
Precision filters
Filter through millions of papers by title, author, journal, publication date, and more. Our advanced search capabilities allow for precise queries, helping you locate specific papers or explore broader topics efficiently.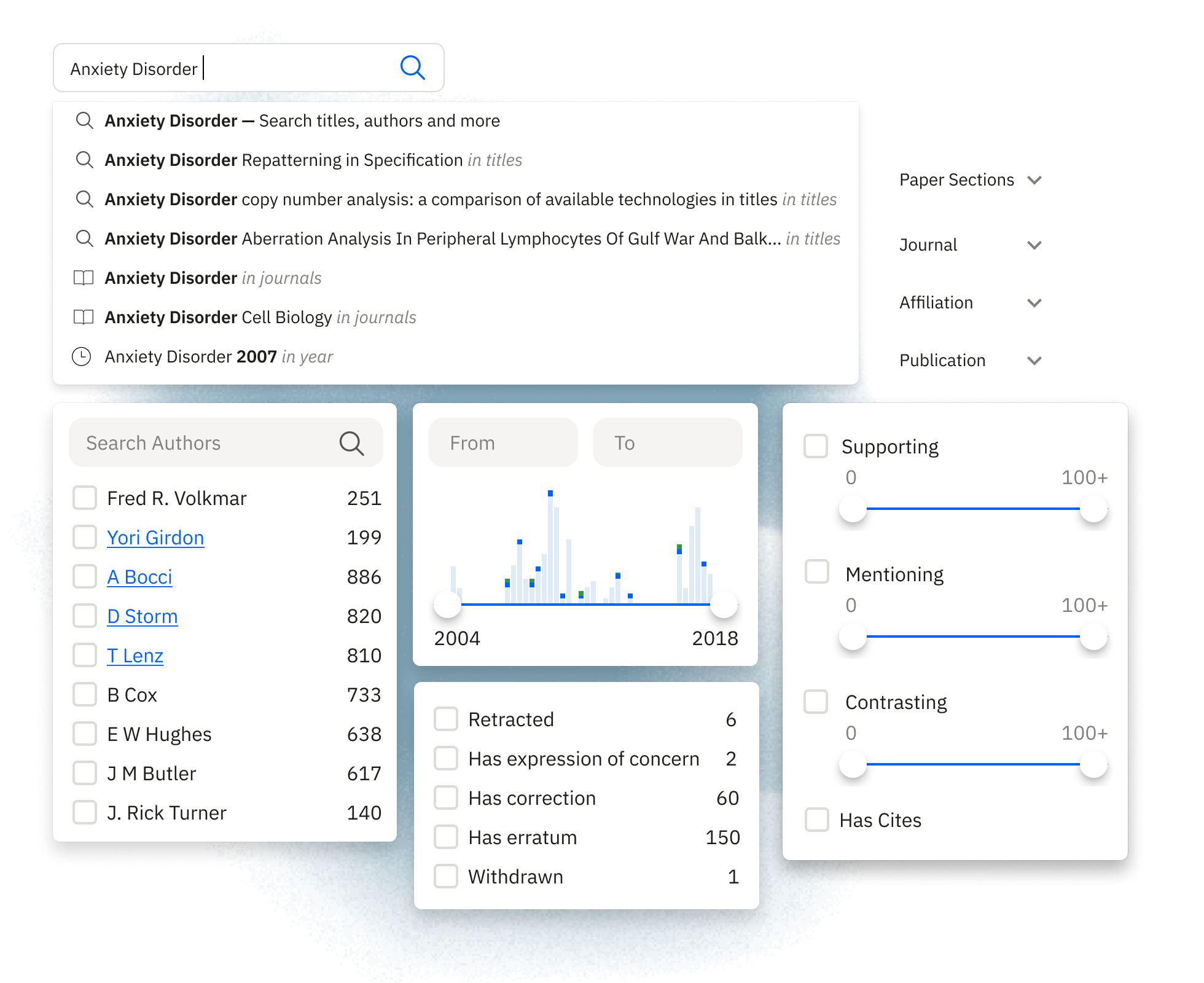
-
Smart Citations
Citations with context
Smart Citations go beyond traditional metrics, showing how papers are cited — whether they're supported, contrasted, or merely mentioned. This context-rich approach helps you quickly evaluate a paper's influence and reception in the scientific community, enabling more informed research decisions.Learn More
AI built for research
Instantly access research insights with our AI-powered Assistant. Leveraging over 1.2 billion Smart Citations, it provides evidence-based answers, streamlining literature reviews and helping you explore new topics efficiently.
Learn More

Precision filters
Filter through millions of papers by title, author, journal, publication date, and more. Our advanced search capabilities allow for precise queries, helping you locate specific papers or explore broader topics efficiently.

Citations with context
Smart Citations go beyond traditional metrics, showing how papers are cited — whether they're supported, contrasted, or merely mentioned. This context-rich approach helps you quickly evaluate a paper's influence and reception in the scientific community, enabling more informed research decisions.
Learn More
Unified Access to Any Scholarly Content
Get Instant access to the research you need from one platform. Buy, rent, or connect your existing journal subscriptions and save up to 30% with a system of record.
Unified Article Access

Copyright Compliant
Pay-Per-Article

“Research Solutions has been a big time-saver for our researchers and a cost-saver for the organization.”
Dan Dowd, Chief Clinical Officer, Genomind
Manage your literature library
Centralize your literature library, collaborate, and share content across your company.
Shared notes and annotations for collaboration
Personal and shared folders for easy organization
Content tagging for easy filtering
Usage insights and access controls for admins
Streamlined billing for all purchased content
Import existing content for centralized management
Analyze and evaluate research
Next-generation tools built to help researchers evaluate and track research easier than ever before.